STRONTIUM
STRONTIUM
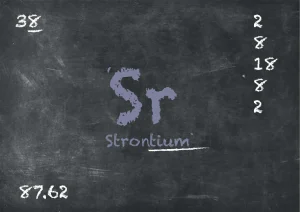
What is strontium?
Strontium, with its atomic number 38 and chemical symbol Sr, belongs to the group of alkaline earth metals. It was first discovered by Adair Crawford in 1790. This soft and silver-coloured metal possesses interesting properties, including its ability to burn in air and react with water.
In addition to its industrial applications, strontium has found uses in the field of medicine, particularly in medical imaging studies. By incorporating strontium into imaging agents, healthcare professionals can obtain clearer and more detailed images for diagnostic purposes.
Moreover, a specific form of strontium, known as strontium ranelate, has shown promising benefits for bone health. Strontium ranelate has been associated with improving bone density and reducing the risk of fractures, making it a valuable option in the management of conditions such as osteoporosis.
Researchers in Poland conducted a study in 2021 to explore the potential benefits of strontium in bone formation and osseointegration. The local administration of strontium was found to significantly improve osseointegration in bone implants, enhancing their stability and long-term success.
Strontium showed promise in supporting bone health and potentially benefiting individuals with osteoporosis, a condition characterised by weakened bones. Further research is needed to fully understand strontium's mechanisms and its clinical applications, but these findings highlight its potential in enhancing bone health and implant integration.
Strontium is a naturally occurring chemical element found in various geological and environmental settings. It is typically found in the Earth's crust associated with minerals and ores. Some common forms and elements in which strontium naturally occurs include:
- Celestite: Celestite (strontium sulfate) is one of the primary minerals containing strontium. It is often found in sedimentary rocks and marine environments.
- Strontianite: Strontianite (strontium carbonate) is another mineral that contains strontium. It is typically found in carbonate-rich geological formations.
- Barite: Strontium can substitute for some of the barium ions in barite (barium sulfate) minerals. As a result, strontium can be present in barite deposits.
- Igneous Rocks: Strontium is found in various igneous rocks, including granites and basalts. It can replace calcium in the crystal structure of certain minerals in these rocks.
- Groundwater: Strontium can leach from rocks and minerals into groundwater, leading to its presence in some underground water sources.
- Plants and Animals: Strontium can be absorbed by plants and animals from the environment and incorporated into their tissues. This can have implications for strontium isotope analysis in ecological and geological studies.
- Nuclear Fallout: In the past, strontium-90 (^90Sr), a radioactive isotope of strontium, was released into the environment during nuclear weapon tests and nuclear accidents, leading to its presence in soils and environmental samples.
Strontium is found in various products, materials, and applications across different industries due to its chemical properties and unique characteristics. Here are some common products and areas where strontium can be found:
- Fireworks: Strontium compounds, such as strontium nitrate and strontium carbonate, are used in fireworks to produce red flame colours. Strontium is responsible for the vivid red hues seen in many fireworks displays.
- Cathode Ray Tubes (CRTs): In older television and computer monitors, CRTs used strontium compounds in the screen's phosphor coating to produce the images. Newer display technologies have largely replaced CRTs.
- Glow-in-the-Dark Products: Strontium aluminate-based materials are used in the production of glow-in-the-dark items, including glow sticks, watch dials, and exit signs. These materials store energy and emit visible light in the dark.
- Flares and Pyrotechnics: Strontium compounds are used in flares and pyrotechnic devices to produce bright red and orange flames.
- Glass Manufacturing: Strontium carbonate is used as a flux in the glass industry to improve the optical quality and colour of glass. It can also reduce the melting temperature of glass batches.
- Pyrotechnic Signal Flares: Strontium nitrate is used in signal flares for maritime and aviation applications.
- Paint and Pigments: Strontium chromate and other strontium compounds are used in some types of paints and pigments, especially those with corrosion-resistant properties.
- Nuclear Medicine: Strontium-89 (^89Sr) is used in radiation therapy for the treatment of bone metastases and certain types of cancer. It targets the bones due to its similarity to calcium and emits beta radiation.
- Drilling Fluids: Strontium compounds may be used in drilling fluids in the oil and gas industry to control wellbore stability and reduce formation damage.
- Ceramics: Strontium compounds can be used in ceramics to achieve specific properties, such as increased hardness and improved electrical conductivity.
- Fire Safety: Strontium carbonate is sometimes used as a fire-retardant additive in materials like plastics and coatings.
- Electronics: Strontium titanate is used in some electronic devices and components, such as capacitors and varistors.
- Metal Alloys: Strontium can be added to certain alloys to modify their properties, such as grain refinement in aluminium alloys.
- Dental and Medical Imaging: Strontium-based contrast agents have been investigated for use in dental and medical imaging.
- Luminous Paints: Strontium aluminate-based luminous paints are used on watch hands and dials for nighttime visibility.



